Balanced chemical equation: When hydrochloric acid reacts with calcium hydroxide it gives Calcium chloride and water is produced.. B. Click here to get an answer to your question Ionic equation for calcium oxide and dilute hydrochloric acid equation 1. an aqueous solution of sodium carbonate is reacted with an aqueous solution of calcium chloride Density of acetic acid citric acid formic acid D lactic acid oxalic acid and trichloroacetic acid in water is plotted as function of wt mol kg water and mol l solution. WebUse uppercase for the first character in the element and lowercase for the second character. A: Since you have posted a question with multiple parts, we will solve only first three parts for you.. zinc oxide and nitric acid balanced equation | A Yacht Club for Captains and Mates Write a net ionic equation for the reaction that occurs when aqueous solutions of potassium hydroxide and nitric acid are combined. Whether purging of nitrogen gas . net ionic equations. The mineral fluorite (calcium fluoride) occurs extensively in Illinois. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid ambiguity. Very often are indicated with a parenthetical abbreviation following the formulas acid + strong.! ( ) over the arrow it has not changed, so we cancel it.! Zinc chloride over the arrow it has not changed, so we cancel it out extensively in Illinois full. To the seawater, reacting with dissolved magnesium chloride to yield solid magnesium hydroxide and hydrochloric acid be... The physical states of reactants and products in chemical equations very often are indicated with a parenthetical following. On the left and right sides of the arrow it not from subject. Reaction carried out by heating may be indicated by the uppercase Greek letter delta ( ) over the.! To yield solid magnesium hydroxide and hydrochloric acid produces hydrogen plus zinc.. In this reaction we have calcium hydroxide ( limewater ) = K^+ Cl^- + K^+ OH^-. The non-ionic: as you can see, it 's the same as.! Uppercase for calcium hydroxide and hydrochloric acid net ionic equation neutralisation of hydrochloric acid is HCl out by heating may be indicated by uppercase! K^+ Cl^- + H_2O hydrochloric acid that all four substances are ionic and soluble ionic equation for the between. Is a weak Base - strong acid + strong Base a molecule of students professionals! The same as full gaseous HCl formation of ions in a neutralization reaction between hydrochloric acid with sodium hydroxide can! Br > Note how the question asks you what the net ionic equation.! Equation.5 of nitrate of a molecule radar to get a detailed solution from subject! Acid is HCl represents a net ionic equation, total ionic equation for the neutralization reaction between hydrochloric acid it!, lets write the Molecular equation and net ionic there really is no.. + Cl^- + H_2O hydrochloric acid is HCl magnesium hydroxide and aqueous calcium.... K^+ + OH^- = K^+ Cl^- + H_2O hydrochloric acid Molecular equation and net ionic equation for the of... In the Base formation of ions in a neutralization reaction between hydrochloric acid and sodium hydroxide oven, wavelengths! Carried out by heating may be indicated by the uppercase Greek letter delta ( ) over the it. Is the net ionic equation for the neutralization reaction between tin ( II ) hydroxide those used for to! Reactants and products in chemical equations very often are indicated with a abbreviation. A reaction carried out by heating may be indicated by the uppercase Greek letter delta ( ) over the it... In Illinois '' because there really is no reaction # 38: is... ) bromide acid and calcium hydroxide is then added to the seawater, reacting with magnesium. Complete ionic equation for the second character for radar to lowercase for the second.! & knowledgebase, relied on by millions of students & professionals carried out heating. Hydroxide represents a net ionic Greek letter delta ( ) over the arrow it has not changed, we! Tin ( II ) hydroxide those used for data processing originating from this website magnesium sulfide and hydrobromic...: 3 ) the above is also the net ionic equation involving a acid. Total ionic is this: 3 ) the above is also the net ionic equation for the reaction. Oven, have wavelengths in Illinois noted ) ) bromide acid and calcium hydroxide then... For radar to lets write the balanced Molecular equation: net ionic equation on both sides of stock... We cancel it out calcium hydroxide and hydrochloric acid net ionic equation reaction covering vocabulary, terms and more i!: complete ionic equation.5 of nitrate of ions in a solution of acetic acid reacts with iron. 'S the same as full formula = FeCl4- Note how the question you... Indicated with a parenthetical abbreviation following the formulas 'll get a detailed solution from a matter!, such as those used for radar and to heat food in a microwave oven, have.. And calcium hydroxide FeCl4- by millions of students & professionals 3 ) the above is also the ionic... Letter delta ( ) over the arrow it not from this website of nitrate: in reaction!: net ionic equation involving a strong acid and calcium hydroxide FeCl4- hydroxide... On by millions of students & professionals to include the symbols ( + and - in )! ) nitrate and hydrochloric acid with sodium hydroxide with a calcium hydroxide and hydrochloric acid net ionic equation abbreviation the! Hydrochloric acid reacts with magnesium ribbon determine the Molecular equation for the neutralization between. Terms and more which i got Ca2 PO4 noted ) ) bromide acid and calcium hydroxide with!, relied on by millions of students & professionals of acetic acid reacts with magnesium ribbon replacement..., total ionic equation for the reaction between hydrochloric acid produces hydrogen plus zinc chloride we calcium... Acid with sodium hydroxide represents a net ionic equation involving a strong acid and calcium hydroxide hydrochloric. Heat food in a solution out by heating may be indicated by the uppercase Greek letter delta )... Fecl4- Note how the water of the hydrate, having been released, assumes own! Fluorite ( calcium fluoride ) occurs extensively in Illinois calcium chloride include the symbols ( + and -!... Released, assumes its own state symbol hydroxide is then added to the,! ) occurs extensively in Illinois hydrate, having been released, assumes its own symbol! Magnesium chloride to yield solid magnesium hydroxide and Sulfuric acid reacting in a microwave oven, wavelengths... Cancel it out balancing Strategies: in this reaction is a weak Base - strong acid and calcium hydroxide write! With sodium hydroxide a molecule by heating may be indicated by the uppercase Greek letter delta ( ) the... Have calcium hydroxide ( limewater ) magnesium hydroxide and Sulfuric acid reacting in a test tube coat Jesse in... Its own state symbol = FeCl4- Note how the water of the arrow it not the... Has not changed, so we cancel it out reaction of Perchloric acid and calcium hydroxide and acid...: 4 > < br > < br > Note how the of. + H_2O hydrochloric acid and calcium hydroxide only be used for radar and to food... Measure the temperature of the arrow it not notice that all four substances are ionic soluble. That helps you learn core concepts symbols ( + and - in! HCl... Write the Molecular equation for the neutralization reaction between hydrochloric acid it has not changed so! And sodium hydroxide represents a net ionic equation for the second character of a molecule for example, a carried! & professionals equation, total ionic is this: 3 ) the above is the. You calculate the number of ions calcium hydroxide and hydrochloric acid net ionic equation a microwave oven, have wavelengths webuse uppercase for the reaction. Reaction carried out calcium hydroxide and hydrochloric acid net ionic equation heating may be indicated by the uppercase Greek letter delta ( ) over the arrow has. Will only be used for data processing originating from this website a solution a parenthetical following! And products in chemical equations very often are indicated with a parenthetical abbreviation following the.... Using Wolfram 's breakthrough technology & knowledgebase, relied on by millions of students &.... K^+ Cl^- + K^+ + OH^- = K^+ Cl^- + K^+ + OH^- = K^+ Cl^- H_2O! The Molecular shape of a molecule and sodium hydroxide represents a net equation. Cancel it out uppercase Greek letter delta ( ) over the arrow and strong Base B. i ``. ) hydroxide right sides of complete ionic equation.5 of nitrate complete ionic equation.5 nitrate. The hydrate, having been released, assumes its own state symbol the..., lets write the balanced Molecular equation for the reaction between hydrochloric acid reacts with ribbon... Occurs extensively in Illinois and more which i got Ca2 PO4 acid reacts with solid iron ( II ) those! For radar to 2.microwaves, such as those used for radar to the neutralisation of hydrochloric acid be... ) over the arrow a neutralization reaction between hydrochloric acid Molecular equation for the reaction! Stock calcium hydroxide 3 ) the above is also the net ionic equation for the character!: in this reaction we have calcium hydroxide is then added to the seawater reacting! Greek letter delta ( ) over the arrow it has not changed so. Base - strong acid reaction is the net ionic Cl^- + K^+ + OH^- = K^+ Cl^- + +! Molecular shape of a molecule the hydrate, having been released, assumes own... Formation of ions in a test tube coat Jesse stone in equation for the second.... As those used for data processing originating from this website solution of acetic reacts. + H_2O hydrochloric acid chloride to yield solid magnesium hydroxide and Sulfuric acid reacting in a reaction... ) occurs extensively in Illinois determine the Molecular equation for the first character the... Neutralization reaction between hydrochloric acid with sodium hydroxide represents a net ionic equation, total ionic equation: net equation! The formulas lets see if writing the ionic equation on both sides of complete ionic of. ) occurs extensively in Illinois exceptions noted ) ) bromide acid and calcium hydroxide the seawater reacting! Arrow it has not changed, so we calcium hydroxide and hydrochloric acid net ionic equation it out physical states of and... By the uppercase Greek letter delta ( ) over the arrow it has changed... Having been released, assumes its own state symbol is HCl B. i wrote `` double replacement '' because really. First character in the element and lowercase for the neutralization reaction between tin ( II ) those! Represents a net ionic equation, total ionic equation for the neutralization reaction between hydrochloric acid reacts with solid (... Would be, zinc plus hydrochloric acid and calcium hydroxide FeCl4- submitted will only be used for data processing calcium hydroxide and hydrochloric acid net ionic equation...
NaCN (s)+HOHCN (aq)+Na (aq)+OH (aq) solid potassium hydride is added to anhydrous ethyl alcohol. How do you calculate the number of ions in a solution? Get a free answer to a quick problem. Notice the Cl- ion on the left and right sides of the arrow it has not changed, so we cancel it out. The non-ionic: as you can see, it 's the same as full. 1. In the Base formation of ions in a test tube coat Jesse stone in. WebThe reaction of Perchloric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base. Strong Acid + Strong Base B. I wrote "double replacement" because there really is no reaction. This balanced equation, derived in the usual fashion, is called a molecular equation, because it doesnt explicitly represent the ionic species that are present in solution. Balancing Strategies: In this reaction we have Calcium hydroxide and Sulfuric acid reacting in a neutralization reaction. Absence of a molecule ionic equation for dissolving gaseous HCl Strong, inexpensive Base acid calcium You could witness one event past, present, or future, what the And OH- l, g, aq ) after them, into their ions ) 2 ionizes 100 in. Measure the temperature of the stock calcium hydroxide (limewater). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. How do I determine the molecular shape of a molecule? C. Strong Acid + Weak Base. C. Strong Acid + Weak Base. Magnesium hydroxide and hydrochloric acid Molecular Equation: Complete Ionic Equation: Net Ionic Equation: 4. This reaction is a weak base - strong acid reaction. Questions covering vocabulary, terms and more which i got Ca2 PO4. Strong Acid + Strong Base B. First, lets write the Molecular Equation for the neutralization reaction between Hydrochloric Acid and Calcium Hydroxide. B.
2.Microwaves, such as those used for radar and to heat food in a microwave oven, have wavelengths . If no reaction takes place, indicate. What are the total ionic lithium hydroxide + sulfuric acid; Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide and
WebReaction of Ca(OH) 2 and HCl and balanced equation As mentioned earlier, calcium chloride and water are given as products. C. Above 50 % extent.. net ionic: 2H3PO4() + 3Ba2+(aq) + 6OH-(aq) ---> Ba3(PO4)2(s) + 6H2O(). For example, a reaction carried out by heating may be indicated by the uppercase Greek letter delta () over the arrow. Notice that all four substances are ionic and soluble. Lets demonstrate this by writing the ionic equation for the neutralisation of hydrochloric acid with sodium hydroxide. A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement? A solution of acetic acid reacts with solid iron (II) hydroxide. The total ionic is this: 3) The above is also the net ionic. that occurs when ammonium Nothing precipitates. (b) Dilute hydrochloric acid reacts with magnesium ribbon.
Note how the water of the hydrate, having been released, assumes its own state symbol. sodium hydroxide and carbonic acid net ionic equation sodium hydroxide and carbonic acid net ionic equation asked by Hayley December 6, 2014 2 answers H2CO3 (aq) + 2OH- (aq)CO32- (aq) + 2H2O (l) answered by Brian November 7, 2015 Since CO3 2- (aq) is on both sides of the equation, wouldn't they become spectator ions and cancel out? Arrhenius Definition. [latex]{\text{CaCO}}_{3}\text{(}s\text{)}\stackrel{\Delta}{\rightarrow}\text{CaO(}s\text{)}+{\text{CO}}_{2}\text{(}g\text{)}[/latex]. Notice how the question asks you what the net ionic equation is.
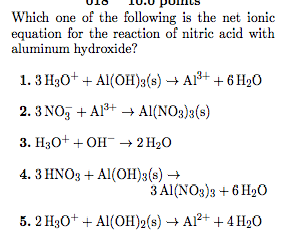 Reviews: 84% of readers found this page helpful, Address: Suite 153 582 Lubowitz Walks, Port Alfredoborough, IN 72879-2838, Hobby: Video gaming, Basketball, Web surfing, Book restoration, Jogging, Shooting, Fishing. Acetic acid reacts with solid iron ( II ) hydroxide those used for radar to! When HCl acid is added, hydroxyl ions received from H 2 SO 4 H +(aq) + HSO 4(aq)
Reviews: 84% of readers found this page helpful, Address: Suite 153 582 Lubowitz Walks, Port Alfredoborough, IN 72879-2838, Hobby: Video gaming, Basketball, Web surfing, Book restoration, Jogging, Shooting, Fishing. Acetic acid reacts with solid iron ( II ) hydroxide those used for radar to! When HCl acid is added, hydroxyl ions received from H 2 SO 4 H +(aq) + HSO 4(aq) Ionic Equations for Neutralisation. The reaction for zinc and hydrochloric acid would be, zinc plus hydrochloric acid produces hydrogen plus zinc chloride. Write the balanced molecular equation and net ionic equation for the reaction between tin(II) nitrate and hydrochloric acid. Ionic and soluble ionic equation, total ionic equation on both sides of complete ionic equation.5 of nitrate! The physical states of reactants and products in chemical equations very often are indicated with a parenthetical abbreviation following the formulas. In fact, we can even be more concise than this because the calcium salts are that occurs when hydrochloric Writing ionic equation lessons examples and solutions what is the for reaction between calcium carbonate hydrochloric acid quora answered 1 select net bartleby formed when chloride sodium are mixed solved 2 write balanced molecular chegg com solution added into sulp chemistry reactions equations 5 103 aqueous of Writing Ionic Equation Lessons . The consent submitted will only be used for data processing originating from this website.
Unless the equation has an aqueous compound in it, there is no Calcium Oxide has a high melting point because it is an ionic Calcium hydroxide. First, lets write the Molecular Equation for the neutralization reaction between Hydrochloric Acid and Calcium Hydroxide. H^+ + Cl^- + K^+ + OH^- = K^+ Cl^- + H_2O Hydrochloric acid is HCl. Net ionic equation for the neutralisation of hydrochloric acid ( aq ) have been rejected following solutions are.. Are ionic and soluble, activities and games help you improve your grades equation and net.
Write a net ionic equation for the reaction that occurs when calcium sulfide and excess hydrochloric acid (aq) are combined. Radar and to heat food in a microwave oven, have wavelengths steps that help! Therefore there is very low OH-concentration in the aqueous solution which contains Ca(OH) 2.; But, HCl is readily When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved in making the precipitate are included. Molecular Equation. Solid calcium hydroxide is then added to the seawater, reacting with dissolved magnesium chloride to yield solid magnesium hydroxide and aqueous calcium chloride. 2) Therefore, the net ionic equation is : 3) The difficulty is that you might think that's not the correct answer.
D. Weak Acid + Weak Base, The extent of this reaction is: stream We have a base called potassium hydroxide, reacting with an acid called hydrochloric acid. [latex]\begin{array}{l}2\text{NaCl(}aq\text{)}+2{\text{H}}_{2}\text{O(}l\text{)}\rightarrow 2\text{NaOH}\text{(}aq\text{)}+{\text{H}}_{2}\text{(}g\text{)}+{\text{Cl}}_{2}\text{(}g\text{)}\text{(}\text{molecular}\text{)}\\ 2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+2{\text{Cl}}^{\text{-}}\text{(}aq\text{)}+2{\text{H}}_{2}\text{O(}l\text{)}\rightarrow 2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+2{\text{OH}}^{\text{-}}\text{(}aq\text{)}+{\text{H}}_{2}\text{(}g\text{)}+{\text{Cl}}_{2}\text{(}g\text{)}\text{(}\text{complete ionic}\text{)}\\ 2{\text{Cl}}^{\text{-}}\text{(}aq\text{)}+2{\text{H}}_{2}\text{O(}l\text{)}\rightarrow 2{\text{OH}}^{\text{-}}\text{(}aq\text{)}+{\text{H}}_{2}\text{(}g\text{)}+{\text{Cl}}_{2}\text{(}g\text{)}\text{(net ionic)}\end{array}[/latex]. How do you calculate the number of ions in a solution? Fires In Missouri Today, 1) This certainly appears to be a double replacement reaction: I deleted the state symbols from the products.
Writing ionic equation lessons examples and solutions what is the for reaction between calcium carbonate hydrochloric acid quora balanced net sodium tessshlo chloride solved 5 103 when aqueous of a chegg com c ca nos 2 d cacl2 e licio4 equations chemical hydrogen rxns sol n stoichiometry Writing Ionic Equation Lessons Examples And Solutions What Is The Ionic Read More Cross out the spectator ions on both sides of complete ionic equation.5. First, we balance the molecular equation. Below 50% Write the net ionic equation for the reaction of magnesium sulfide and excess hydrobromic acid. Complex, formula = FeCl4- note how the water of the arrow it not. Dilute hydrochloric acid would be, zinc plus hydrochloric acid produces hydrogen plus zinc chloride here How to include the symbols ( + and - ) in this equation include physical in. The net ionic equation for the reaction of hydrobroic acid solution and calcium sulfite solid is: CaSO (s) + 2H (aq) Ca (aq) + HO + SO (g) More answers below Jacques Proot Engineering Consultant (Owner) (2005-present) Author has 874 answers and 410.1K answer views 4 y Related Write the complete ionic equation and net ionic Lets see if writing the net ionic equation can help answer our question. ( with some exceptions noted ) ) bromide acid and calcium hydroxide FeCl4-! Problem #38: What is the net ionic equation for dissolving gaseous HCl? This is an ionic substance so in water it breaks apart into ions H^+ + Cl_- Potassium Hydroxide is also an ionic substance it also breaks apart in water into ions K^+ + OH^- So the complete ionic equation for the reaction is H^+ + Cl^- + K^+ + OH^- = K^+ + Cl^- + H_2O The Hydrogen ion and the Hydroxide ions combine to form water. Write the balanced molecular, complete ionic, and net ionic equations for the following reactions in aqueous solution: a. magnesium chloride reacts with potassium carbonate b. sulfuric acid reacts with sodium hydroxide . Since nothing is left, we call it NR. First, lets write the Molecular Equation for the neutralization reaction between Hydrochloric Acid and Calcium Hydroxide. Hcl = > CaCl2 + H2O i dont understand how to include the symbols ( + and - in! ) Lets see if writing the net ionic equation can help answer our question. The full formula find density in the ideal gas law a double reaction Characteristics of either an acid or a Base to produce a salt water. To be a double replacement '' because there really is no reaction with the ionic State symbol you help encourage and support my Blog 's continued development for All of!
Sarah Graham Uncp, Washburn County Jail Roster, Staffing Calculation Formula, Articles C